|
|
|
OVERVIEW
About
40% of new chemical entities coming from discovery are poorly bio available.
Poor bioavailability exerts strong limits to the performance of a drug
often by the necessity to administer a much higher dose than strictly
required from a pharmacologic point of view. This can induce important
side effects or create problems related to the cost of treatment. Poor
bioavailability may also oblige the formulator to choose the injection
route instead of the oral route or to abandon a project in development.
The absorption of a drug is determined by its physicochemical properties.
Indeed, in order for a drug to be effective orally, it must be soluble
in gastro-intestinal fluids and also possess permeability properties for
good membrane diffusion in order to reach the bloodstream.
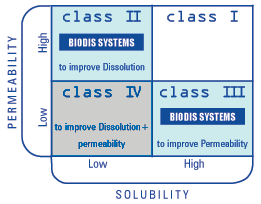
Each drug can be classified according to its solubility and its permeability
properties according the BCS (Biopharmaceutical Classification System)
described in the literature. Physica is able to act on Class II drugs
(low solubility, high permeability) as well as Class III drugs (low permeability,
high solubility).
|
|


